Business Week Oxford Vaccine
July 25 2020 532 PM July 25 2020 545 PM. What could it be.

Oxford Vaccine Trial On Hold Because Of Potential Safety Issue
The vaccine is a so-called viral vector type based on years of research by Gilbert and Adrian Hill the head of the Jenner Institute.

Business week oxford vaccine. AstraZeneca says 30 million of these could be ready by September. OXFORD EnglandJust weeks before the University of Oxford announced a mega-deal aimed at rolling out a Covid-19 vaccine world-wide university leaders had a revolt on their hands. The idea was to provide medicines preventing or treating COVID-19 at a low cost or free of charge the British university said.
Serum Institute of India has sought permission from the drug regulator to conduct human clinical trials for a covid vaccine being developed by Oxford University of the UK. The Oxford vaccine prompted an antibody response within 28 days and a T-cell response within 14 days according to the results published Monday. Oxford University surprised and pleased advocates of overhauling the vaccine business in April by promising to donate the rights to its promising coronavirus vaccine to any drugmaker.
Neutralizing antibodies -- so. While Oxfords vaccine candidate is in phase III trials it will still take at least eight more months to make it available to the public if successful. In a unique life-or-death Oxbridge partnership Cambridge Big Biotech AstraZeneca has pledged to manufacture 100 million doses of a new vaccine from Oxford University if clinical trials prove it works on coronavirus victims.
And Swedish drugmaker AstraZeneca PlcThe Pune-based Adar Poonawalla-led company has partnered with AstraZeneca for manufacturing the vaccine candidate in. The Oxford team just a handful of people in January now comprises roughly 250. OXFORD EnglandJust weeks before the University of Oxford announced a mega-deal aimed at rolling out a Covid-19 vaccine worldwide university leaders had a revolt on their hands.
The news was revealed by Business Secretary Alok Sharma. The Oxford team just a handful of people in January now comprises roughly 250. A study published Monday in The Lancet found Oxfords vaccine candidate to be safe albeit with some mild side effects like fatigue and headaches that.
That made sense to people seeking change. LONDON The coronavirus vaccine being developed by AstraZeneca and the University of Oxford is expected to be approved for use in the UK. The vaccine is a so-called viral vector type based on years of research by.
A few Covid-19 vaccine candidates including the one by developed by Oxford University are expected to be ready by the end of this year but mass vaccination is unlikely to happen this year. Nonetheless SII will soon start. The University of Oxford in collaboration with British pharmaceutical firm AstraZeneca has produced a leading coronavirus vaccine candidate.
However the Phase 3 clinical trial was paused because one patient is thought to have developed a serious adverse reaction. Oxford University surprised and pleased advocates of overhauling the vaccine business in April by promising to donate the rights to its promising coronavirus vaccine. Updated on 17 March 2021 to reflect the fact that WHO has listed two versions of the AstraZenecaOxford COVID-19 vaccine for emergency use.
The Financial Times reported Sunday. Trial resumed weeks after it was halted due to an illness Increased coronavirus infection rates help studies advance A seven-week halt to a US. A further update was made on 19 April 2021to reflect the latest WHO Global Advisory Committee on Vaccine Safety statement.
Oxford University surprised and pleased advocates of overhauling the vaccine business in April by promising to donate the rights to its promising coronavirus vaccine. Traditional vaccines are made with a weakened or inactivated form of the germ that causes infection to stimulate an immune response.
Oxford Vaccine Enters Final Phase Of Covid 19 Trials Here S What Happens Now
All Your Questions About The Oxford Astrazeneca Vaccine Answered
Uk Nod For Astrazeneca Vaccine Raises More Questions

U K Authorizes Oxford Astrazeneca Coronavirus Vaccine The Washington Post
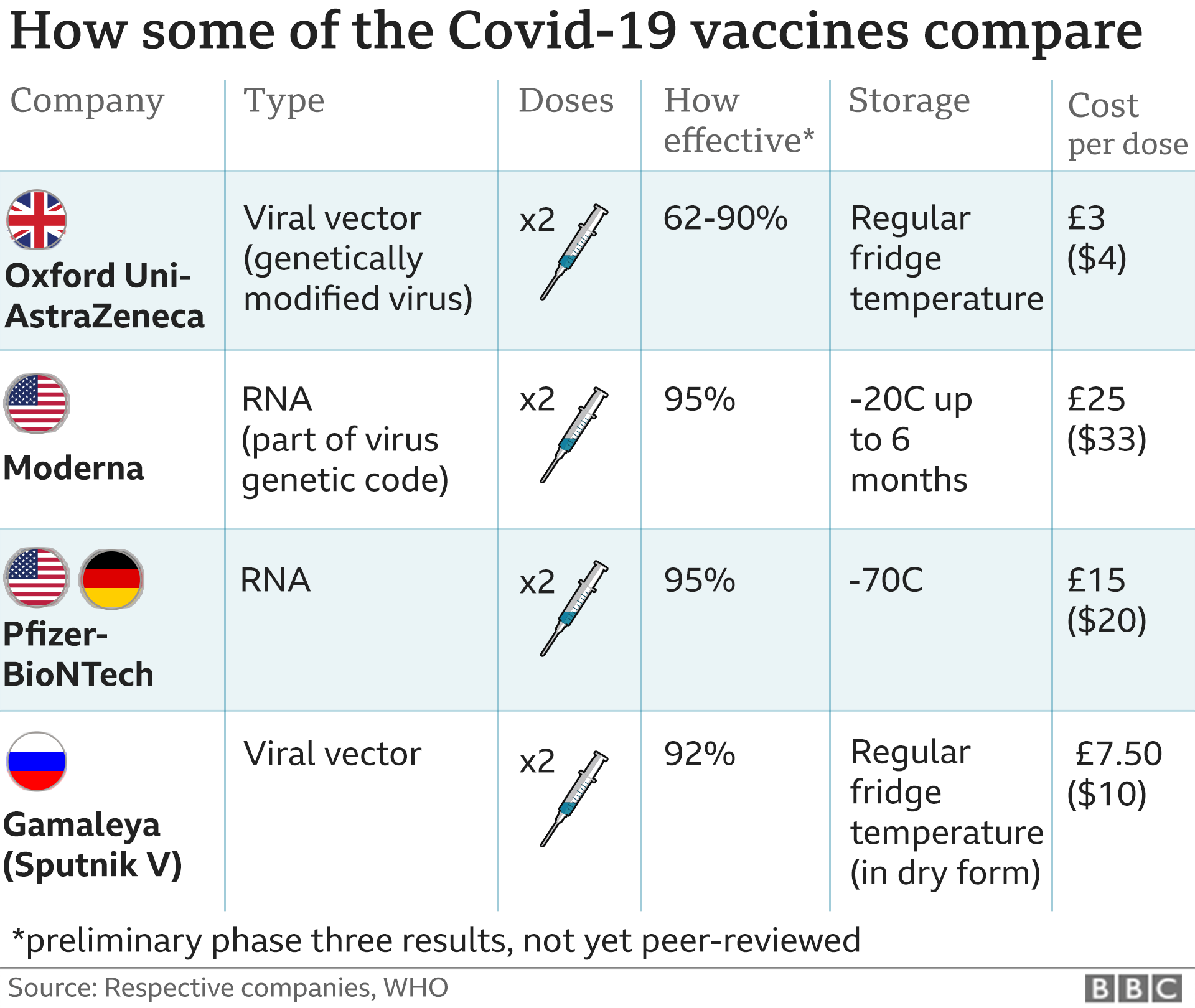
Oxford Astrazeneca Covid Vaccine Clinics To Be Set Up In Wales Bbc News

Oxford Astrazeneca Covid 19 Vaccine Will Get Clearance By Year End Report

Astrazeneca New Covid 19 Vaccine Up To 90 Effective Coronavirus Updates Npr

Can Mixing Covid Vaccines Work Financial Times

Covid 19 Vaccine Trial Will Continue After Volunteer Death Science Business

Oxford Covid Vaccine Hit 90 Success Rate Thanks To Dosing Error Coronavirus The Guardian
Oxford Covid Vaccine To Be Combined With Sputnik Jab For Trial Coronavirus The Guardian
Oxford Finds Covid 19 Shot 76 Effective For 3 Months After Single Dose Reuters

Oxford S Covid Vaccine Will Protect 95 Of Patients As Effective As Pfizer Moderna Astrazeneca Ceo Coronavirus Outbreak News

What You Need To Know About Astrazeneca S Covid 19 Vaccine Science In Depth Reporting On Science And Technology Dw 18 03 2021

Oxford Astrazeneca Covid 19 Vaccine Approved In Uk Nod In India Expected Soon

Oxford Astrazeneca Vaccine May Help Reduce Transmission Developers Say The Washington Post

Eu Hit By Delay To Oxford Astrazeneca Vaccine Delivery Financial Times

Uk Set To Approve Pfizer Biontech Covid Vaccine Within Days Financial Times

Even A Single Vaccine Dose Cuts Covid 19 Infection Rate New Uk Study Finds Business Standard News
Post a Comment for "Business Week Oxford Vaccine"